It also forms part of all living beings but is rarely found in the earth’s crust. It is an odorless, colorless, and tasteless gas. Final hint, this is an inert gas responsible for the different colors of the aurora. I think you would have guessed the answer by now. Yes, we are talking about Nitrogen. It is the most abundant gas found in the earth’s atmosphere and forms around 78.1% of our air, i.e., four-fifths of the earth’s atmosphere. Nitrogen is also an important part of proteins and nucleic acid found in all living organisms. It is responsible for the orange-red, blue-green, blue-violet, and deep violet colors of the aurora. Nitrogen was discovered by Scottish physician Daniel Rutherford, British chemist Henry Cavendish, and British clergyman Joseph Priestley around the same time in 1772. However, the credit for its discovery is given to Rutherford as his study was published first. Rutherford called nitrogen unhealthy air. In 1790 Jean-Antoine-Claude Chaptal suggested the name nitrogene for the element as it was found in nitric acid and nitrates. It was finally named nitrogen after the Greek word nitron, meaning “native soda”, and genes meaning “forming,” It is considered to be the seventh most abundant element in the Milky Way galaxy and the fourth most abundant element in the human body that, contains around 3% nitrogen by mass. However, Nitrogen content in the Earth’s crust is very scarce, around 0.3 parts per 1,000. In space, free nitrogen is known to occur on many meteorites, in some stars and nebulae, as well as in the sun. On the earth’s surface, it is found in the volcano gases, mines, and mineral springs. Another material found in Bat’s caves, named guano, is also rich in nitrogen. Liquid nitrogen appears as a colorless liquid and is very cold, with a temperature of around − 320 °F. It causes frostbite if it comes in direct contact with the skin. The liquid nitrogen container may rupture violently upon prolonged exposure to heat. So, isn’t this a unique element? Are you ready to learn more about it? In the coming sections, we will discuss the properties and uses of nitrogen in detail. So keep reading.
Properties of Nitrogen
Nitrogen is a non-metal element located in the P block of the Periodic table. It is the seventh element of the periodic table and a member of group 15 and period 2. The elements of this group are also referred to as pnictogens, and nitrogen is the lightest member. Nitrogen has the atomic number 7 and is denoted by the chemical symbol N. It is a colorless, odorless, tasteless, and inert gas that usually occurs in a diatomic state. It has 5 electrons in its valence shell, due to which most of its compounds are trivalent in nature. It has the ability to form hydrogen bonds as well as coordination complexes with other elements. There are two stable isotopes of nitrogen 14N and 15N. Amongst these 14N forms around 99.634% of natural nitrogen, while 15N forms only 0.366%. It is a non-toxic and non-combustible gas but does not support life. An excessive amount of nitrogen in the atmosphere can be fatal for living organisms. A few important properties of nitrogen are mentioned in the table given below:
Uses of Nitrogen
Applications of nitrogen are quite varied. Let us discuss the important uses of this element in this section. 3rd: 4578.1 kJ/mol
Commercial Uses
• Food Preservative
Nitrogen delays rancidity and other oxidative damage to food, due to which it is used in food packaging and preservation. It is a safe and inert gas that has replaced oxygen and other supplement gases in the food preservation industry. It helps protect the nutrients and freshness of food and prevents microbial growth.
• Incandescent lights
Nitrogen is also used in incandescent light bulbs as an alternative to argon gas because, being an inert gas, it does not react with the hot filament of the bulb. Also, it reduces the evaporation of tungsten from the filament giving long life to the bulb.
• Fire suppressant system
Nitrogen is also used as a fire suppressant for IT equipment. It works by reducing oxygen content to the extent that the fire douses off itself. Also, it does not produce any byproducts when exposed to a flame.
• Manufacturing stainless steel
Nitrogen has replaced carbon in the manufacturing of high-strength austenitic stainless steel as it has greater solid solubility, improves pitting corrosion resistance, is a strong austenite stabilizer, and also works as a potent interstitial solid-solution strengthener.
• Hardening of steel
The low carbon, low alloy steel does not harden easily, due to which nitrogen is added. This helps overcome problems such as case carburizing.
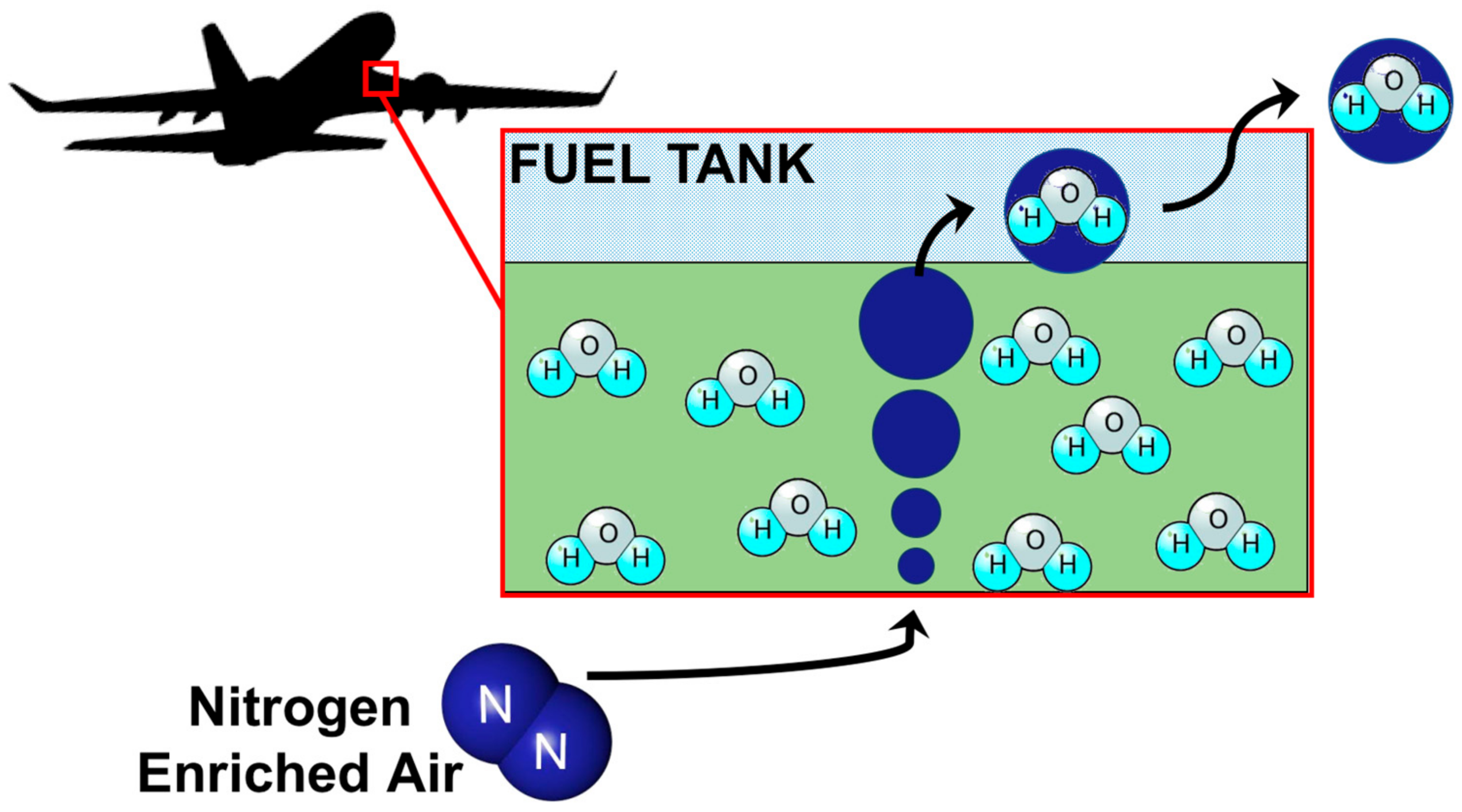
• Aircraft fuel system
Nitrogen gas is denser than oxygen at room temperature. Hence, it is used in the fuel system of aircraft to replace oxygen from the tank, also known as nitrogen purging. This helps in preventing fire or explosions in the fuel tank.
• Tires of aircraft & race cars
Pure nitrogen is one of the best heat insulators that is filled in the tires of race cars and aircraft, where it controls the temperature rise and gives long life to tires.
• Euthanasia
As an asphyxiate gas, nitrogen is also used in executing capital punishment in certain countries instead of the lethal injections used earlier.
Laboratory Use
• Sample preparation
Nitrogen is used in chemical analysis for the preparation of samples. It is used to concentrate the liquid samples by reducing their volume. This is done by directing the pressurized stream of nitrogen gas perpendicular to the surface of the liquid sample. This causes the solvent to evaporate, leaving behind a concentrated solution with more amount of solute.
• Cryopreservation
Pure liquid nitrogen at a temperature of about −320°F (−160°C) is used for preserving biological material such as microbes, cells, tissues, etc., for the long term as it provides ultra-low temperature. These can be stored in cryopreservation without loss of viability. It is also used in the preservation of blood as well as the reproductive cells, i.e., eggs and sperm.
• Cold trap
Liquid nitrogen is used in making cold traps in laboratories. It is used for condensing gases onto a cold surface. This helps prevent unwanted gases from reaching those areas of the system where they can cause damage or malfunction. The gases are removed from the chamber with the help of a vacuum pump, after which they are transferred to the cold trap, where they get condensed.
Industrial Uses
• Hydraulic system
Nitrogen is considered one of the safest gases to be used in a hydraulic accumulator system. Being an inert gas, it does not react with hydraulic oil under pressure. It is used in the accumulators to keep the hydraulic fluid pressurized.
• Construction Equipment
Pressurized nitrogen also provides extra power to construction equipment such as hydraulic hammers.
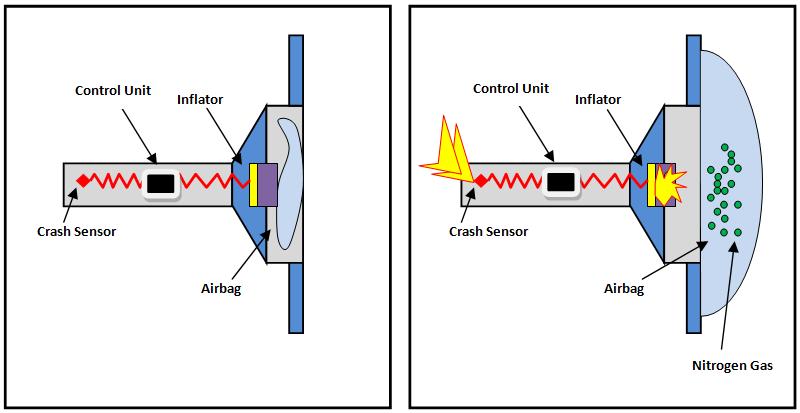
• Airbags
Airbags in vehicles utilize pressurized nitrogen produced from the decomposition of sodium azide (NaN3). When a car crashes, the trip sensors signal to an ignitor. This result in the generation of heat which causes the sodium azide inside the airbags to decompose into sodium metal and nitrogen gas that fills up the airbags.
• Dyes
A large synthetic organic class of dyes known as azo dyes contains nitrogen as an azo group (˗N=N˗). These are widely used in the paint, fiber, textile, leather, and cosmetic industry. Besides their primary use of coloring, these dyes are also cytotoxic, antifungal, antiviral, and antibacterial agents.
Medicinal Uses
• Cryotherapy
It is a treatment used to remove cysts, warts, or abnormal tissues from the body. Usually, liquid nitrogen or argon gas is used for this purpose. It is also used for the treatment of cancer. Due to its low boiling point, low price, and ease of use, liquid nitrogen is considered the best freezing agent and is used in cryotherapy worldwide.
Miscellaneous Uses
• Heat-sensitive equipment such as infrared and X-ray detectors are cooled using liquid nitrogen. • Liquid nitrogen is also used for freeze grinding and machining soft or rubbery materials at room temperature. • It is used in plumbing works, such as to halt pipes flow when valves are not present or when construction or repair work is in progress. • Liquid nitrogen is used in the freeze branding of livestock. Few Related topics Uses of Argon Uses of Boron Uses of Titanium Uses of Selenium Uses of Silicon Uses of Helium Uses of Chlorine
Conclusion
Nitrogen is used for various purposes, such as in the preservation of food, incandescent lights, fire suppression systems, manufacturing and case hardening of steel, cryopreservation, hydraulic systems, construction equipment, vehicle airbags, cryotherapy, etc. Happy Reading.