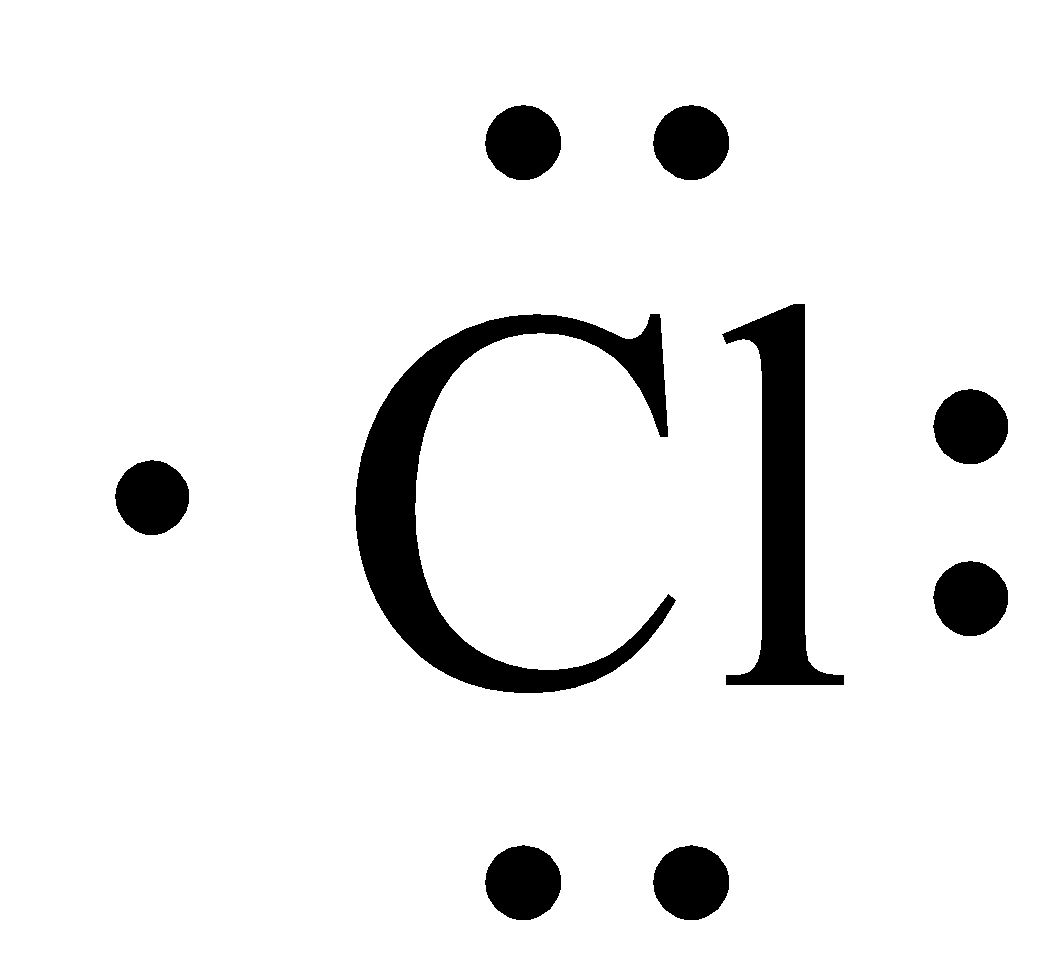Also, free chlorine also occurs in volcanic gases. Chlorine is used for the purpose of sanitization, disinfection as well as antisepsis. Chlorine gas was also used as a weapon in World War I. Welcome, I always love to share stuff about chemistry. If you have an interest in the element chlorine, will show you how to draw a Bohr diagram of chlorine using step by step guide. Keep reading this article to the very end for knowing how to make a Bohr diagram of elemental chlorine in a simplistic way. So, let’s begin our discussion without any delay.
Bohr Model of Chlorine
The Bohr-Rutherford model was presented by Niel Bohr in 1913. The original Rutherford model was unable to explain certain properties exhibited by various atoms due to which Bohr made certain corrections in the existing model and the final theory was postulated which is considered quite accurate even to date. The Bohr model of an atom is used to illustrate the atomic structure for an element in a pictorial manner displaying all the atomic species viz. protons, neutrons, and electrons. To appreciate the Bohr-Rutherford model we must first get acquainted with a few important terms listed below: • Nucleus: It is the core of the atom and exhibits a positive charge due to the presence of protons. It is positioned right at the center of the atom and contains protons and neutrons. • Neutrons: These are the charge-neutral atomic species that are located inside the nucleus of the atom. Usually, neutrons are symbolized as n°. • Protons: These are also located inside the nucleus and carry a positive charge. Protons are denoted using the symbol p+. • Electrons: These are the only atomic particles that are present outside the nucleus. Unlike protons and neutrons that remain stationary inside the nucleus, electrons move in a definite path around the nucleus. They carry a negative charge and are distributed around in the nucleus in accordance with their energy. • Shells: The circular path taken by the electrons around the nucleus is known as shell or orbit. Each shell is allowed to accommodate a certain number of electrons with definite energy. As the energy of every shell is fixed these are also known as energy levels. As per the Bohr model, the shells are named as K, L, M, N, etc., or 1, 2, 3, 4, etc. The number increases away from the nucleus as the energy also increases in the same direction. The electrons located in the shell closest to the nucleus have minimum energy and are said to be in the ground state. The electrons are allowed to jump from lower to higher energy levels as they absorb energy and also, drop down from higher to lower energy levels as they release energy. The outermost energy level or shell of an atom is known as the valence shell. The electrons located in the valence shell participate in chemical bonding between two or more atoms of the same or different elements. The chlorine atom contains 18 neutrons, 17 protons, and 17 electrons. The electrons revolve around the nucleus in K, L, and M shells.
Drawing Bohr Model of Chlorine
Chlorine is a halogen element located in group 17 of the periodic table.
The information that we can derive from the above-mentioned Chlorine box is as follows: • The atomic number of chlorine is 17. • The electronic configuration of chlorine is [Ne] 3s23p6. • The chemical symbol of chlorine is Cl. • The atomic mass of chlorine is 35.353. To draw the Bohr model of chlorine we will first have to find out the atomic species contained in this atom. Let us first start with determining the number of protons in chlorine. The number of protons for any atom is equal to the atomic number of that atom. In the case of the chlorine atom, the atomic number is 17. Therefore, for the chlorine atom, the number of protons = atomic number = 17 Now, we will calculate the number of neutrons present in the chlorine atom. For any atom number of neutrons present in the nucleus is given by the following formula: Number of neutrons = Atomic mass (rounding it up to the nearest whole number) – Number of protons As mentioned in the chlorine box drawn above the atomic mass of the chlorine atom is 35.353. After rounding it up to the nearest whole number we get 35. Now, put the values in the above-mentioned formula: Number of neutrons in chlorine atom = 35 – 17 = 18 As protons and neutrons are present in the nucleus of an atom, we can draw the nucleus of a chlorine atom by incorporating the above values.
In the above diagram, p+ is used to symbolize protons, and n° is used to symbolize neutrons. Now, in order to complete the Bohr model of chlorine, we will have to find out the number of electrons in the atom. For any atom, the number of electrons is always equal to the atomic number of that atom. Therefore, in the case of chlorine atoms, Number of electrons = Atomic number of chlorine = 17 Moving forward to the next step, we now have to accommodate these electrons in their respective energy level. For this, we need to understand that every shell has a limited capacity to accommodate the electrons. The maximum number of electrons that can be housed in a particular shell is given by 2n2. In this formula, n denotes the number of shells. Now, we will step by step calculate the number of electrons for every shell of the chlorine atom. For the K shell of the chlorine atom, the maximum number of electrons = 2 (1)2 = 2 After adding these two electrons to the first shell the atom appears as follows:
Now, to add the second shell to the atom we will have to calculate the maximum number of electrons for it. The maximum number of electrons for L shell of chlorine atom = 2 (2)2 = 8 Hence, the L shell has the capacity to house 8 electrons. However, one important point to be mentioned at this stage is that the electrons to any shell, other than the K shell, are always added in a group of four in a clockwise direction. The first four electrons added to a shell are placed at a 90° angle to each other. This angle keeps on reducing as the number of electrons increases in a shell. So, for the L shell of the chlorine atom, we will first add 4 electrons after which the atom is drawn as follows:
The remaining four electrons are now added to the L shell of the chlorine atom, again in a clockwise manner. After adding all the eight electrons to the L shell, the chlorine atom is now represented as:
Thereafter, we are left with 7 more electrons which will now be accommodated in the M shell. We will first calculate the maximum number of electrons that can be housed in the M shell: The maximum number of electrons for M shell of chlorine atom = 2 (3)2 = 18 Hence, 18 electrons are allowed to exist in the M shell. As we are left with 7 electrons, we will first add four electrons in a clockwise manner. The chlorine atom now looks as follows:
Now, finally adding all the seven electrons to the shell after which it appears as follows:
Hence, the final Bohr model of the chlorine atom consists of 18 neutrons and 17 protons in the nucleus, and 17 electrons revolving in the group of 2, 8, and 7, in the K, L, and M shell, respectively. Related Topic Silicon Bohr Model Boron Bohr Model Sodium Bohr Model
Deriving Lewis Structure from Bohr Model
Lewis structure of an atom displays the valence electrons present in an atom in a pictorial manner. The atoms are represented by their chemical symbol while the electrons are illustrated as dots due to which the Lewis structures are also referred to as Electron dot diagrams. As discussed in the previous section, the outermost shell of the chlorine atom is the M shell which contains 7 electrons. These are the valence electrons of the chlorine atom. Hence, the Lewis structure for chlorine atom is given as under:
Properties of Chlorine
A few important properties of chlorine are given below: • Chlorine is a non-metal, halogen element. • It is a highly reactive element that exists as a greenish-yellow gas at room temperature. • The melting and boiling points of chlorine are -103 °C and -35 °C, respectively. • The density of chlorine at 1 atm pressure and 0°C temperature is 3.214 gm/L. • It is a highly electronegative element and exists in a number of oxidation states viz. -1, +1, +3, +5, and +7.
Conclusion
As per the Bohr model of the chlorine atom, it consists of 17 protons, 17 electrons, and 18 neutrons, respectively. The neutrons and protons reside inside the nucleus and electrons orbit around the nucleus in definite circular paths called shells. The number of protons, as well as the number of electrons in an atom, is always equal to the atomic number of that atom. The number of neutrons is given by the formula: Number of neutrons = Atomic mass (rounding it up to the nearest whole number) – Number of protons The Bohr model of chlorine contains 3 shells viz. K, L, and M shell containing 2, 8, and 7 electrons respectively. The maximum number of electrons that can be housed in a shell is given by the formula 2n2, where n is the number of shells.