Melting is a physical process in which the transfer of phase from solid to liquid takes place. The melting point is the temperature at which the solid and liquid can coexist in equilibrium. It depends on various factors like external pressure, presence or absence of impurities, etc. The kinetic energy of particles in the solid state is less than in the liquid state. On heating a solid substance, the kinetic energy of particles increases, and as a result, the solid structure breaks down to form a liquid. The temperature remains constant during the process of melting as the heat is being utilized in changing the state. Chemical changes generally involve either application of heat or the release of heat. In this article, we will study these two types of reactions and classify melting as exothermic or endothermic. So, is melting exothermic or endothermic? Melting is an endothermic process as we need to apply external heat to a solid substance in order to make it melt. Heat is absorbed by the reactant species in the melting process and the change in enthalpy results out to be positive indicating the reaction to be endothermic. We will study the fundamentals of the melting process and endothermic reactions. Stay connected!
What is the Enthalpy Change?
The heat content of a system is called enthalpy. Heat is a form of energy. The energy changes during the reaction can be expressed by a change in enthalpy. Change in enthalpy can come out to be positive or negative. Change in enthalpy is positive if heat is absorbed and negative if heat is released during the reaction.
What is an Endothermic Reaction?
The word “endo” has been derived from the Greek language, and it means “inside.” It is a type of chemical reaction in which reactants absorb heat from the surroundings to form the products, or we can say in this reaction, heat is taken inside by the reacting species. Heat is provided to the reactants from external sources. The change of enthalpy will be positive, which means that the product’s energy is more than the energy of reactants. Lower is the energy, stable are the species. Here, the reactant species are more stable. For example, photosynthesis is an endothermic reaction as it uses the sun’s energy to make food for plants. Since energy is taken from outside, it is an endothermic reaction.
What is an Exothermic Reaction?
The word “Exo” has been derived from the Greek language, which means “outside”. It is a type of chemical reaction in which heat is released along with products, or we can say that it is a reaction in which heat is taken outside from the species. No heat is provided to the reactants from external resources. The change of enthalpy will be negative, which means that the product’s energy is less than the energy of reactants. Lower is the energy, stable are the species. Here, the product species are more stable. The given diagram is called a reaction coordinate diagram. In this, the energy of species is plotted as a function of reaction progress. It represents an exothermic reaction. For example, respiration is an exothermic process. Respiration is defined as the oxidation of food to release energy. Since energy is released, it is an exothermic process.
Why is Melting an Endothermic Process?
A process can be classified as exothermic or endothermic. Without performing actual experiments, we consider a reaction to be endothermic if we need to supply heat to make the reaction occur or if we observe that the species’ temperature has decreased (beakers turn cold). Melting is considered to be an endothermic reaction because we need to supply heat from external sources to facilitate the process. The process won’t take place if the solid is not heated. Substances with low melting points like water can melt slowly without human interference as well, but substances like iron need to be supplied heat in excess amounts. The energy of products is more than the reactants as the product is liquid which has more kinetic energy than solid. For instance, the enthalpy change for melting ice is 6.01kJ/mole.
What happens in an Endothermic Reaction?
The reacting species absorb energy equivalent to the energy required to proceed with the reaction and form a transition state. The transition state then leads to desired product formation. The events during the endothermic reaction can be illustrated using the melting of ice as an example. When ice is heated, the kinetic energy of the constituent atom increases, and they start vibrating. On subsequent heating, the atoms vibrate with greater energy. When the energy absorbed becomes equivalent to the activation energy, the structure of ice starts breaking. Ice and water exist in equilibrium till all ice is converted to water. The temperature remains constant during the melting process. Once all ice has been melted, only the liquid phase exists, and the temperature rises. Boiling can take place on further increasing the temperature.
Properties of an Endothermic Reaction
• It lowers the temperature of the surroundings by absorbing heat. • Enthalpy change comes out to be positive. • These are generally phase-changing reactions or bond-forming reactions. • From the reaction coordinate diagram, we conclude that the activation energy for endothermic is high due to which it needs heat from external sources. Activation energy is the energy required by reactants to proceed with the reaction and form a transition state. The maxima in the curves represent the transition state. • Most decomposition reactions are endothermic reactions.
Factors Affecting Melting Point
Every substance melt at a specific temperature. Most of the time, we have a temperature range instead of a particular temperature. The temperature at which reacting species absorb energy equivalent to the energy required to proceed with the reaction is called the melting point. The melting points of different substances are different because of
• Intermolecular forces
If the intermolecular forces are high, the atoms of a particular solid are closely bound together. This implies that more energy will be required to break the bonds. Higher intermolecular forces imply a higher melting point. That is why ionic compounds have a higher melting point than covalent compounds.
• Dipole Moment
A higher dipole moment implies that the force of attraction between molecules is high and as a result melting point is high.
• Symmetry
In symmetrical packing, molecules are tightly packed, and more energy is required to break the bonds. Hence, symmetrical compounds have higher melting points. This is the reason why neopentane has a higher melting point than n-pentane.
• Size
larger molecules have higher melting points because more energy is required to break them. This is the reason why octane has a higher melting point than methane. The melting point of a particular substance is not definite. It changes with
• Pressure
The relation of pressure with melting point is obtained using a phase diagram. The melting point decreases with increasing pressure for substances with less density in the solid-state than in the liquid state. For those substances which have more density in the solid-state than in the liquid state, the melting point increases for increasing pressure.
• Presence of Impurities
Non-volatile impurities affect the colligative properties, and they can decrease the melting point. The impurities create defects in the lattice and hence we can overcome the intramolecular forces easily. In cold countries where it snows in the winter season, people use salt to change the melting point and clear off roads. You must read out an interesting article on why does salt makes ice colder.
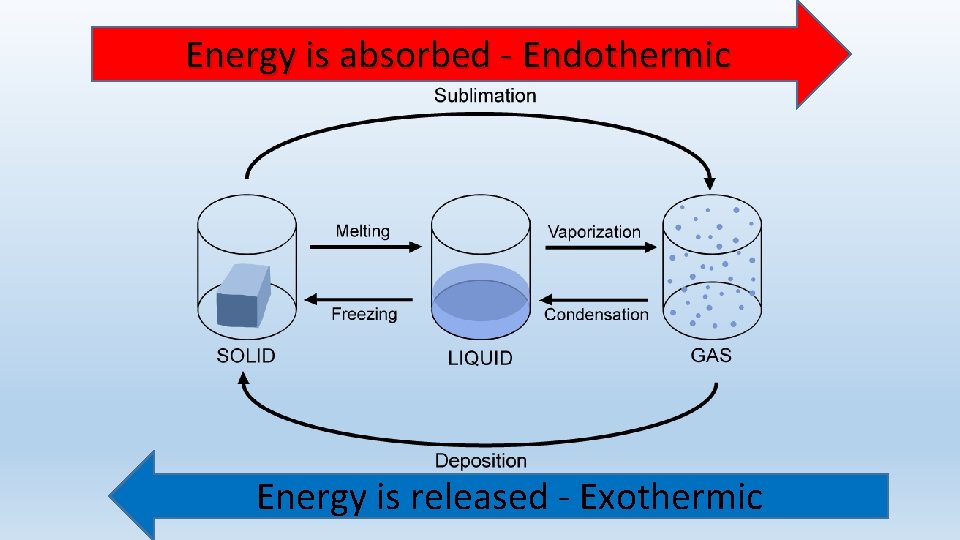
Processes Similar to Melting
1. Fusion
Fusion is the process in which there is a transition in phase from liquid to solid at a particular temperature. It is the reverse of melting. It is an exothermic process. The enthalpy change of the reaction comes out to be negative.
2. Boiling
Boiling is the process in which phase changes from liquid to vapors at a particular temperature. It is an endothermic process. The enthalpy change of the reaction comes out to be positive.
3. Condensation
Condensation is the process in which there is a transition in phase from vapors to liquid at a particular temperature. It is the reverse of boiling. It is an exothermic process. The enthalpy change of the reaction comes out to be negative.
4. Sublimation
Sublimation is the process in which there is a transition in phase from solid to gas at a particular temperature. It is an endothermic process. The enthalpy change of the reaction comes out to be positive.
5. Deposition
The deposition is the process in which there is a transition in phase from gas to solid at a particular temperature. It is an exothermic process. The enthalpy change of the reaction comes out to be negative.
6. Evaporation
Evaporation is the process in which phase changes from liquid to vapors at any temperature. It is an endothermic process. The enthalpy change of the reaction comes out to be positive.
Conclusion
Melting is an endothermic process. We need to supply heat to the solid substance to make it melt. The energy of products is more than that of reactants. Enthalpy change is positive for an endothermic process. Various factors can alter the melting point of a substance. Happy Reading!