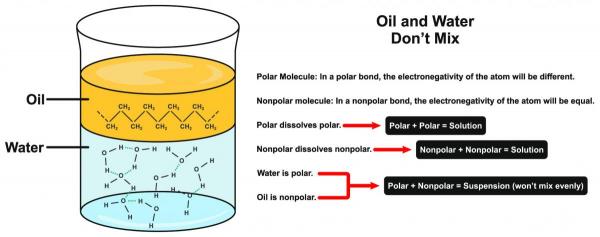
They are used in cooking, medicines, cosmetics, and a number of other industries such as paints, plastic manufacturing, etc. Water on the other hand is a polar substance that is also sometimes referred to as a universal solvent as it dissolves most substances (more than any other solvent). Water finds its use in almost every sphere of life like agriculture, industries, etc. It also forms the main component of the human body. In this article, we will study the fact is oil and water a heterogeneous mixture or homogeneous mixture. So, is oil and water a heterogeneous mixture? Yes, Oil and water form a heterogeneous mixture because these two liquids are immiscible with each other and form a heterogeneous mixture. The intermixing does not occur due to the difference in nature of both the molecules, the oil molecules are non-polar and hydrophobic while water molecules are polar. As they do not blend, the composition of the mixture is not uniform. The two liquids instead form separate layers, also known as phases. Usually, oil is lighter in weight than water and therefore, forms the upper layer while the heavier water molecules settle at the base. The composition and properties of these phases are uniform, as they involve only one type of molecule unless disturbed externally.
Why is oil in the water a Heterogeneous Solution?
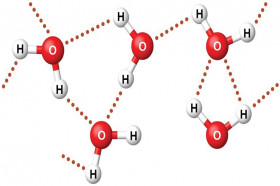
When oil and water are mixed, the oil molecules are hydrophobic in nature, i.e. they hate water, repel away from water molecules. Moreover, the water molecules being polar attract each other and also form hydrogen bonds with each other. This results in the separation of the two liquids which form two different phases, an upper lighter oil layer and a lower heavier layer of water. Have you ever tried to vigorously shake a bottle containing oil and water mixture? If yes, then you would have noticed that the two liquids mix for some time after which they again form separate layers. It happens due to the formation of an emulsion. If oil and water are to be mixed for a long time an emulsifier is required to be added. Oil is a non-polar liquid that is known to be soluble only with other non-polar liquids. Similarly, water is a polar solvent and is found to be immiscible with most non-polar solutions. Therefore, they exist as two separate layers instead of a mixture when placed together. The molecules of both these liquids are unevenly distributed throughout making it a heterogeneous mixture. The two liquids can be easily differentiated in the mixture through visible examination which is also a property of a heterogeneous mixture. To enhance our understanding of this concept let’s try and understand a few terms stated earlier.
What is a Mixture?
A mixture is composed of two or more different types of atoms or molecules which are not combined chemically. The substances which combine to form a mixture do not lose their identity i.e. their original properties remain integral. However, the physical properties of the mixture may still differ from any of its components. The components of a mixture do not require any chemical process for separation and can be easily separated using physical processes such as mechanical or thermal. The mixtures have a variable composition and the enthalpy of mixing i.e. the energy change when a mixture is formed from the pure component is almost negligible as there is no chemical process involved. Mixtures can be solids, liquids, or gases. For example, water and alcohol are liquid in a liquid mixture, smog is a mixture of fog and smoke, alloys are the mixtures of two solids, etc.
Properties of a Mixture
• It is composed of two more different types of atoms or molecules combined in varying ratios. • The uniformity of a mixture might vary from one point to another, in the case of heterogeneous mixtures. For example, the percentage of oxygen in the air may differ from one place to another. • Mixtures may be separated into their elemental composition using physical processes. • The identity or properties of the different components in a mixture are unaffected as no chemical reaction takes place in the formation of a mixture. • The properties of a mixture might be different from one or more of its components. • The properties of a mixture might vary at different points depending upon the occurrence of its constituents. • Usually, mixtures have a range of melting or boiling points instead of a specific temperature. The mixtures can further be broadly divided into homogeneous and heterogeneous mixtures. As discussed before oil in water is an example of a heterogeneous mixture.
What is a Heterogeneous Mixture?
The mixture in which the components (atoms/molecules) are non-uniformly distributed is called a heterogeneous mixture. In such mixtures, the components are separately visible and can be easily distinguished from one another. The components are present in different phases which although are mixed but are still physically separate. Emulsions are a type of heterogeneous mixtures which are formed of two liquid components. A few examples of the heterogeneous mixture are sand in water, ice, and soda, different types of candies mixed, etc.
Homogeneous Mixture
The mixtures in which the components are uniformly distributed throughout the mixture are known as homogeneous mixtures. The components are not visibly distinguishable and may require boiling or similar physical processes for separation into their constituents. The chemical properties of individual components remain unaltered but the mixture in itself may exhibit properties different from any of the components involved. Alloys are a type of homogeneous mixture where one or more components is metal. The components are mixed in a specific ratio. The solutions are also categorized as homogeneous mixtures, which are formed by mixing a solute in a solvent. A few examples of homogeneous mixtures are saltwater, air, alloys, etc.
Homogeneous vs Heterogeneous Mixtures
Homogenization
It is the process of conversion of a heterogeneous mixture of insoluble liquid into a homogeneous mixture. The most common example is homogenized milk (it is also an example of fat in water) where through extensive mixing procedures the fat globules are broken into such small entities that they do not separate from the milk.
Why Oil in Water is not a Homogeneous Mixture?
As discussed earlier uniformity is the main characteristic of a homogeneous mixture. In the case of oil in a water mixture, the two components are incapable of combining evenly with each other. Oil molecules being hydrophobic tend to remain away from water molecules, linked with other oil molecules (lipophilic). Also, water molecules are polar in nature and due to electronegativity difference between oxygen and hydrogen atoms form hydrogen bonds with other water molecules, which keep the water molecules together. Being heavier water molecules sink down and are found at the base of the mixture while oil molecules are present in the top phase.
It is also seen that even after rigorous mixing of these two components (oil and water), they again reorganize themselves as separate identities when left to rest for a while. Resultantly, two separate phases are formed again which are unevenly distributed throughout the mixture. As homogeneous mixtures are characterized by the presence of only one phase, oil in the water definitely cannot be placed in this category. Also, the two phases of this mixture can be easily distinguished through visible examination which is a property of a heterogeneous mixture.
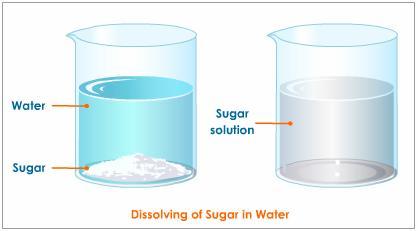
Why is sugar solution homogeneous while oil in water mixture heterogeneous?
When sugar is dissolved in water the sugar particles are spread evenly throughout the solution indicating uniformity of the solution which is the basic characteristic of a homogeneous solution. On the other hand, in the oil in the water mixture, the oil particles are unevenly distributed throughout the mixture. Upon dissolving the sugar molecules in water the solution exists in a single-phase, condition of the homogeneous mixture while oil and water exist as two different phases, condition of a heterogeneous mixture. Once dissolved sugar cannot be differentiated from the solution through visual examination unless properly separated through thermal processing. Oil in water on the other hand is very easily distinguishable.
Conclusion
• Oil in water is a heterogeneous mixture in which the two components form two different phases and are unevenly distributed throughout the mixture. • The oil and water layers are visually distinguishable and can be separated through simple physical processes which is also a property of heterogeneous mixtures. • Mixtures are broadly classified as heterogeneous and homogeneous. Hope you loved the article. If you still have any queries related to the solubility of oil and water, feel free to ask me through comments. Thanks!