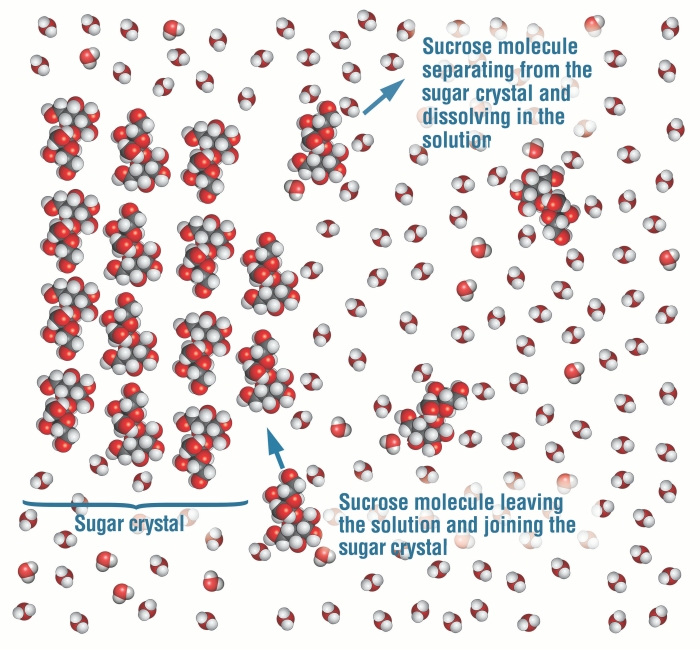
Water is a polar liquid. It is commonly used as a solvent because of its properties of availability and affordability. Also, it is essential for human survival. In this article, we will understand the properties of a mixture formed by mixing these two entities- water and sugar and classify it based on uniformity in chemical composition. So, is sugar water homogeneous or heterogeneous? Sugar water is a homogeneous mixture as sugar completely dissolves in water, and we cannot distinguish between solid and liquid phases. The sugar molecules uniformly get mixed with water molecules forming a homogeneous mixture. The chemical name of table sugar (commonly used in our homes) is sucrose. Sucrose is a disaccharide, and one sucrose unit consists of two monosaccharides, glucose, and fructose. The chemical formula of sucrose is C12H22O11. It is obtained naturally from plants and is a biopolymer.
Why is Sugar Water Homogeneous?
When sugar is added to water, sugar molecules dissolve entirely in water because they are more attracted to water molecules than they are attracted to each other. We cannot distinguish between sugar and water once sugar is dissolved completely. Only one phase can be observed as a sugar solution is formed. A sugar solution is homogeneous.
Why Does Sugar Dissolve in Water?
Dissolve means “to get incorporated” When we add sugar to water or milk, it gets dissolved in the liquid and makes it sweet. It retains its property in the mixture. In a sugar crystal, individual sugar molecules are held together due to the intermolecular force of attraction (weak). When they are added to water, crystals first go to the bottom if we do not stir them. This is because intermolecular forces are still intact, and exposure to water does not have much effect. At this stage, it is a heterogeneous mixture. On stirring the mixture, sugar crystals disappear slowly. It disappears because it gradually gets dissolved in water. On mixing, sugar molecules become attracted to water molecules. Their attraction towards water molecules is much greater than their attraction towards each other. Water molecules can break the bonds between sugar molecules and surround them. Sugar is said to be dissolved when all sugar molecules are surrounded by water, and we cannot distinguish between two phases. Energy is required to break the bonds between two sucrose molecules. The bonds formed at the end compensate for the energy used to break pure solute and pure solvent bonds. Sugar particles get in the spaces of water molecules because intermolecular forces are lesser in liquids than in solids.
What is a Mixture?
A mixture is formed when two or more pure substances are mixed such that they do not form any bonds. The components of a mixture retain their property and can be separated. Mixtures are classified-
- Based on uniformity in composition and appearance • Homogeneous mixture • Heterogeneous mixture 2 Based on particle size • Solution • Suspension • Colloid
What is a Homogeneous Mixture?
The word “homo” means the same. A mixture is homogeneous if the appearance and chemical composition are uniform throughout. We cannot distinguish between various components in a homogeneous mixture. We observe only one phase. Homogeneous mixtures can be confused with pure substances and are called solutions. We cannot get back the mixture’s components by just physical processes. For example, an alloy is a homogeneous mixture of metals or metal and other elements. Brass is an alloy of copper and zinc, and we cannot distinguish copper and zinc in brass.
What is a Heterogeneous Mixture?
The word “hetero” means different. A mixture is heterogeneous if the appearance and chemical composition are not uniform throughout. We can distinguish between various components. We observe two or more phases. We can separate the components of a mixture by physical processes. For example, the mixture of sand and nails is a heterogeneous mixture as we can visually distinguish sand and nails. Homogeneous mixtures can behave as heterogeneous if they are not mixed properly. Classification of mixtures based on particle size • Colloid- A colloid is a heterogeneous mixture with medium-sized particles. The particles are large enough to see but not large enough to settle or be filtered out of the mixture. For example, gelatin, homogenized milk, etc. Read out the article on is milk a homogeneous mixture. • Suspension- A suspension is a heterogeneous mixture with large particles. The particles are large enough to see and settle or be filtered out of the mixture. For example, sand in water, paint, etc. • Solution- A solution is a homogeneous mixture with tiny particles. The particles are too small to see and also too small to settle or be filtered out of the mixture. For example, sugar solution, salt solution, etc. Check out the article on is the salt solution a homogeneous solution.
Why is sugar solution not a heterogeneous mixture?
The sugar solution is not a heterogeneous mixture because we do not see crystals and water separately in sugar water. Only one phase can be observed. Any solution in which a single phase is seen is a homogeneous solution.
How can we separate sugar from sugar solution?
The sugar solution is a mixture, so we can separate the components. We can heat the sugar solution until all water molecules leave the solution. We use the distillation method to separate sugar from water. In this process, different components of the mixture can be separated based on the boiling point of the component in the mixture. For a solid-liquid mixture like sugar solution, we heat the solution till the boiling point of the liquid. The liquid is converted to vapor at this temperature, leaving us with solid. We have a hygroscopic solid, which means that it can absorb water from the surrounding. Sugar starts to decompose at 180°C. The method of obtaining pure sugar crystals is called crystallization. The temperature required for crystallization is much greater than the boiling point of water. Thus, we obtain sugar by heating the solution to the boiling point of water. The solution is heated till all water molecules are converted to vapor. We are left with pure sugar crystals. We can also separate sugar from solution by evaporation as it also involves the conversion of liquid molecules to vapor states. The drawback of using evaporation is that we do not get pure sugar at the end. The sugar so obtained is charred.
Why is sugar solution homogeneous and oil and water heterogeneous?
Water is a polar liquid, while oil is a non-polar liquid. The thumb rule of solubility is “like dissolves like.” Sugar dissolves in water by breaking the bonds between pure sucrose and pure water molecules. In sugar (sucrose), oxygen develops a partial negative charge, and hydrogen develops a partial positive charge due to the bond between the oxygen and hydrogen atoms (O–H bond). Sucrose is a polar molecule as well. The negative and positive regions on the polar sucrose molecules are attracted by the polar water molecules, causing sucrose to dissolve in water. This is the reason because of which sugar in water is a homogeneous solution For the oil to dissolve in water, oil has to break the hydrogen bonds of water. This is not allowed as the bond formed later would not be stable, and energy will not be compensated. Thus, polar water and non-polar oil can not form a homogeneous solution. Oil and water have different densities. Both are liquids, and we can distinguish between the two phases. We can separate water from oil using a separating funnel. Thus, Oil and water form a heterogeneous solution. Read out the write I wrote on is oil and water a heterogeneous mixture.
Fun Fact
The solubility of sugar in water is more at a higher temperature. As the temperature of water increases, the kinetic energy of particles increases, and they move with greater velocity. Their interaction with sugar molecules becomes faster and frequent, due to which they can interact with more and more sugar molecules. There is no change in volume on adding sugar to water as sugar molecules are inserted in the spaces between water molecules.
Conclusion
Sugar water is a homogeneous mixture. It is also called a sugar solution. We cannot distinguish between sugar and water in sugar solution. Sugar gets dissolved in water because sugar molecules are more attracted to water. The force of attraction between sugar molecules is relatively weaker than the force between a sugar molecule and a water molecule. Sugar can be separated from sugar solution by the process of distillation. Oil and water form a heterogeneous mixture because oil is non-polar while water is polar. Oil floats on water as the density of oil is less than the density of water. Happy Reading!